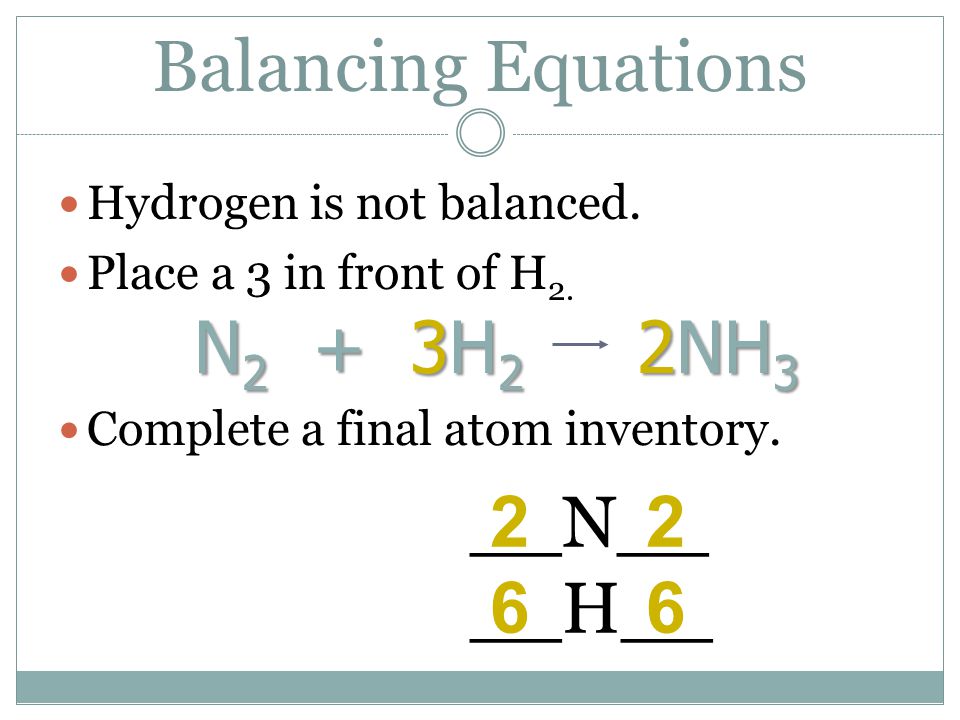
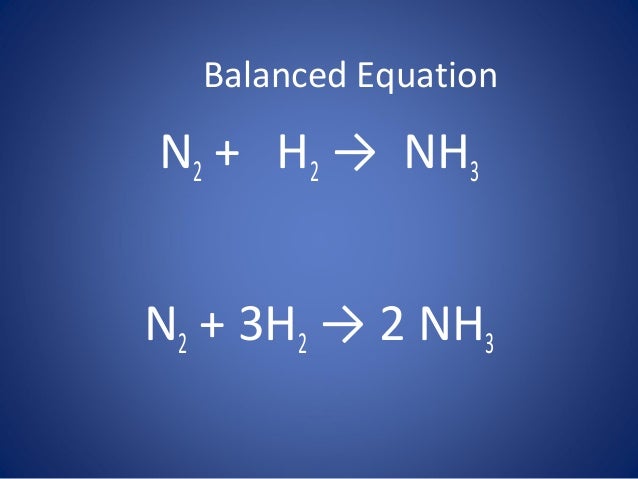
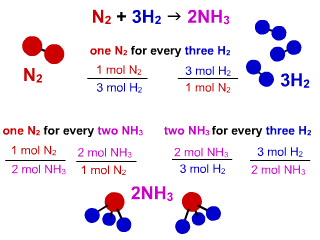
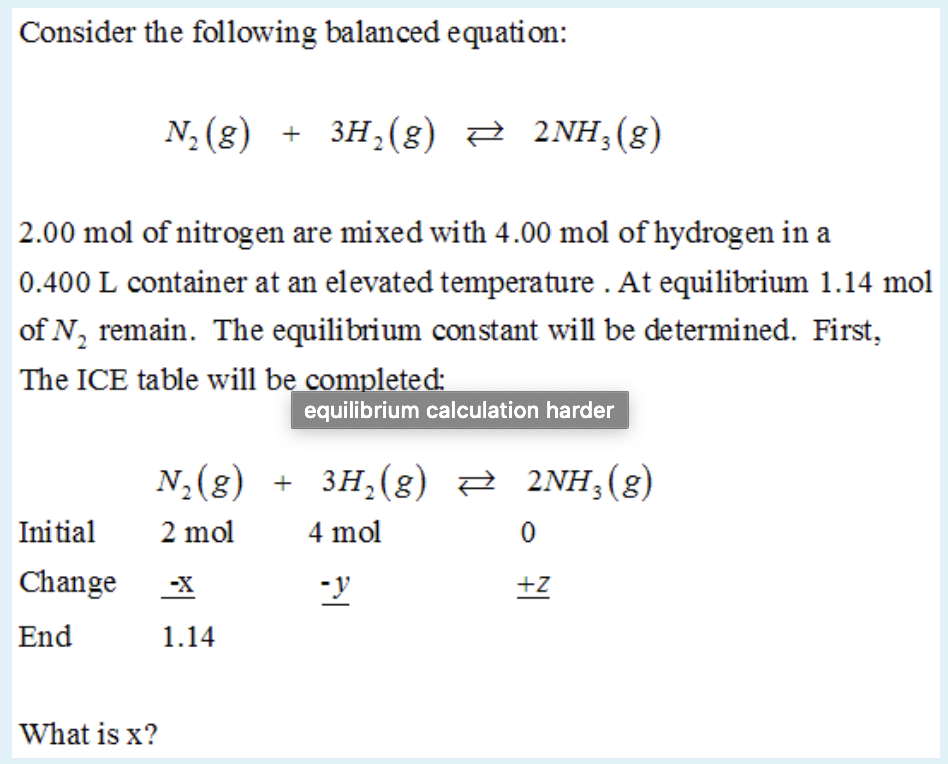
Solved] What is the balanced equation that represents the reaction between hydrogen and nitrogen to make ammonia (NH 3 )? | Course Hero

Bell Ringer Jan. 31 N2 + 3H2 2NH3 How many moles of NH3 are created from 18 grams of H2? How many moles of N2 are needed to create ppt download
Consider the N2(g) + 3H2 (g) - 2NH3 (g), then how many moles of NH3 can be formed from 3.0 Mol N2 and 6.0 mol H2? - Quora
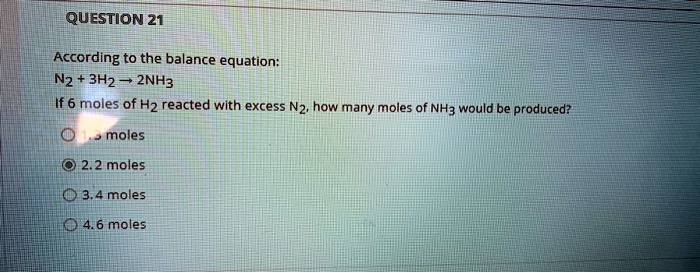
SOLVED:QUESTION 21 According to the balance equation: N2 3H2 2NH3 If 6 moles of Hz reacted with excess Nz, how many moles of _ NHa would be produced? moles 2.2 moles 03.4 moles 014.6 moles