Balance the given equation by oxidation number method - FeSO4 + HNO3 + H2SO4 = Fe(SO4)3 + NO + - Chemistry - Redox Reactions - 13629296 | Meritnation.com
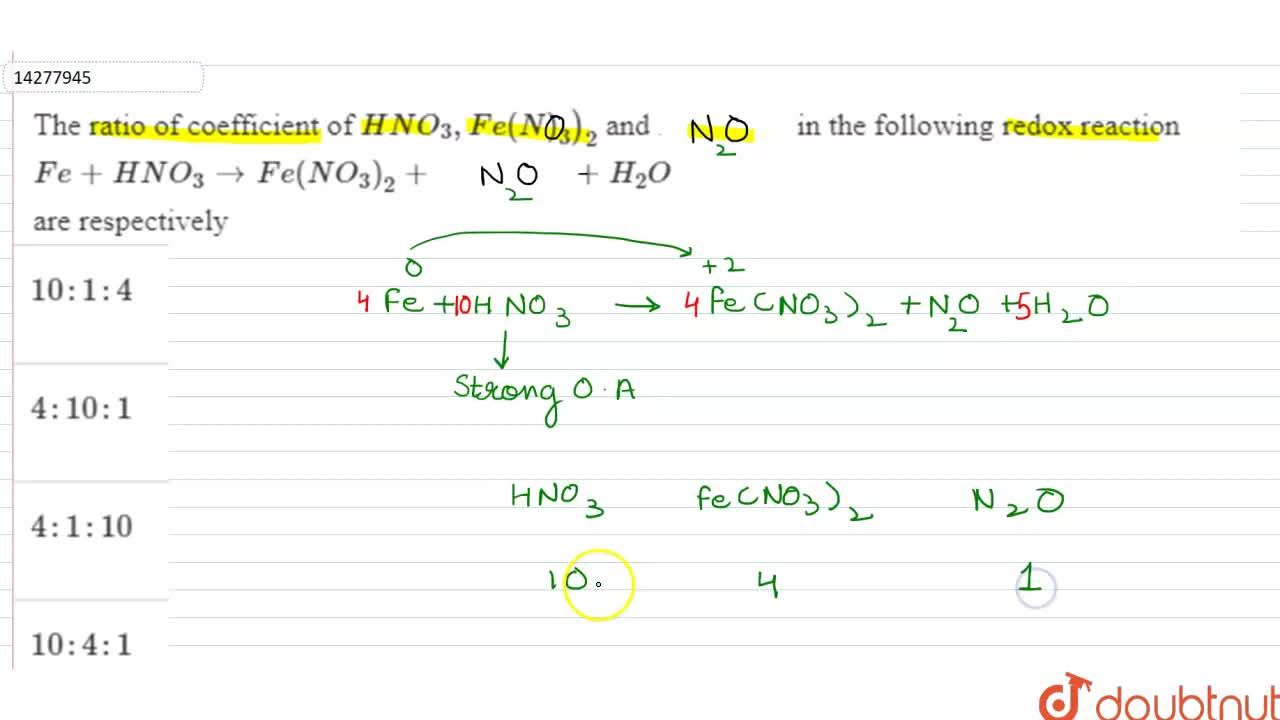
The ratio of coefficient of HNO_(3), Fe(NO_(3))_(2) and NH_(4)NO_(3) in the following redox reaction Fe + HNO_(3) rarr Fe (NO_(3))_(2) + NH_(4)NO_(3) + H_(2)O are respectively

The ratio of coefficient of HNO3,Fe (NO3)2 and NH4NO3 in the following redox equation, Fe + HNO3→Fe (NO3)2 + NH4NO3 + H2O in the balanced form will be?

The ratio of coefficient of HNO3,Fe (NO3)2 and NH4NO3 in the following redox equation, Fe + HNO3→Fe (NO3)2 + NH4NO3 + H2O in the balanced form will be?

OneClass: write a balanced net ionic equation for A. dissolving of Ni (OH)2 in nitric acid. B. Ni 2+ ...

The ratio of coefficient of HNO3,Fe (NO3)2 and NH4NO3 in the following redox equation, Fe + HNO3→Fe (NO3)2 + NH4NO3 + H2O in the balanced form will be?
The reaction of iron with dilute HNO3 gives no reaction due to passivity or gives passivity only? Can anyone explain this to me because I can't understand this reaction in my chemistry

Balance the following chemical equation Fe(s) +H2O(g) = Fe3O4 + H2(g) MnO2 + HCL = MnCl2 + Cl2 + - Science - Chemical Reactions and Equations - 14408537 | Meritnation.com
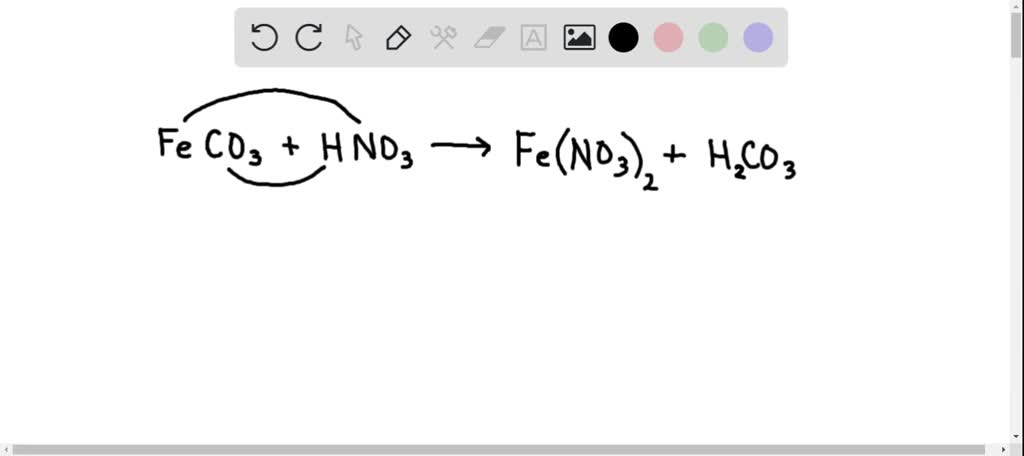
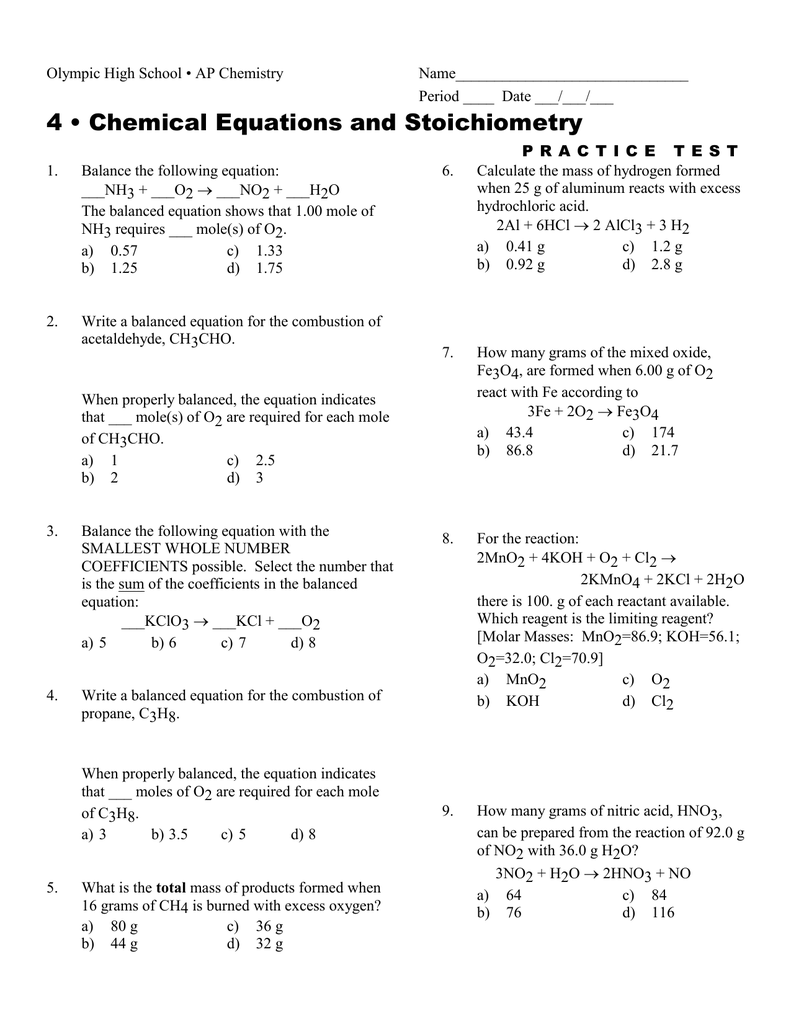
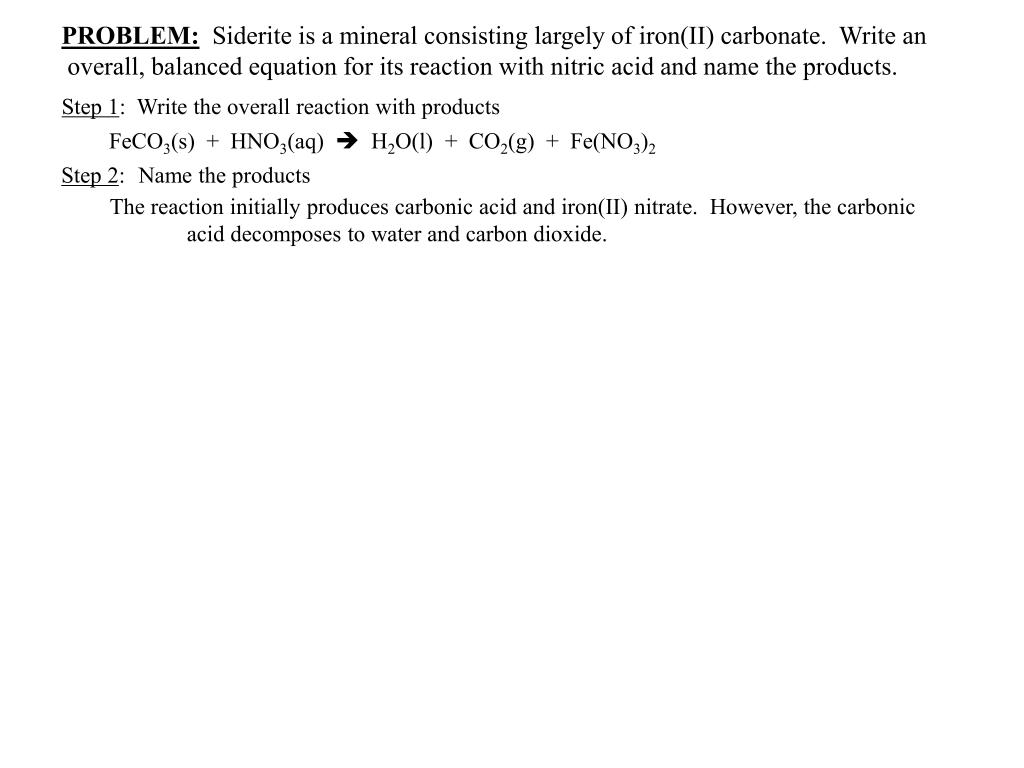
SOLVED:Siderite is a mineral consisting largely of iron(II) carbonate. Write an overall, balanced equation for its reaction with nitric acid, and name the products.





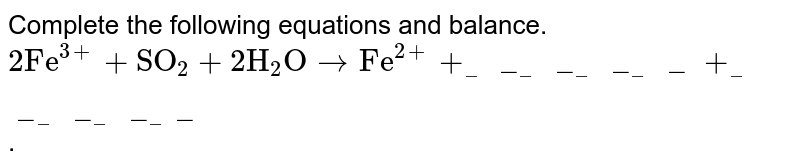


![Balancing Chemical Equations - [DOCX Document] Balancing Chemical Equations - [DOCX Document]](https://demo.vdocuments.mx/img/378x509/reader026/reader/2021092609/55cf91ad550346f57b8f8efd/r-2.jpg)